Water that can start Fire – Supercritical water
A special state of substance that exists above its critical point is known as supercritical fluid
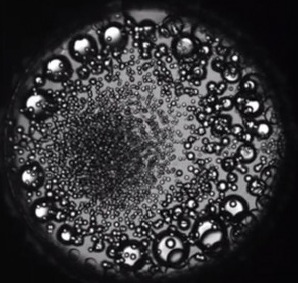
Imagine an indestructible glass ball which is half-full of water, and the rest is a vacuum overhead it. Some of the empty space will be filled by water vapor. If we heat this ball the water boils and forms steam. Thus density of liquid water decreases, while that of steam increases. As we keep heating it, then comes one point, the density of water and steam are equal. This temperature is known as critical point, where both liquid and gas states combine into a state called supercritical water (SCW).
Supercritical Water
Supercritical water( scH2O ). Is water which is put under extreme pressure and heat i.e. 217 atmospheres and 373° C. When water is in its liquid state, water forms many ‘hydrogen bonds’ among its molecules, this makes water expand when freezing, making ice lighter than water. As the water is heated up, the molecules transfer more and more about and the hydrogen bonds breakdown. At the critical point the hydrogen bonds disappear completely.
Many chemicals which are used for making plastics, medicines etc. are not soluble in water because its hydrogen bonds do not allow water molecules to combine with those molecules. Thus solvents like benzene or acetone is used to dissolve them. But when hydrogen bonds are shattered, water molecules can dissolve chemicals that were previously insoluble.
These days in manufacturing decaffeinated coffee powder and for creating nano-materials the supercritical form of carbon dioxide is used.
Supercritical Water may prove to be a useful method for closed-system poop management in space.
One issue is what to do about the salt problem. As water become supercritical, any salts present in the water precipitate out. Thus the system containing the supercritical water face this problem Supercritical Water has well-known reputation for corrosion, second only to that of hydrogen fluoride.
Research in supercritical water is still going on and it will further lead to newer applications of super critical water.